Research in our group focuses on the synthesis, structure, and reactivity aspects of organometallic chemistry with emphasizing on the complexes capable of facilitating small molecules and inert bond activation, performing homogeneous catalysis, and mimicking the organometallic units in enzymes and cofactors. Research involves extensive synthesis, structural analyses with modern spectroscopic and computational methods, and detailed investigations of reactivity. Currently, our group’s research mainly focuses on three projects listed as below:
Low-coordinate transition-metal species, i.e., metal species with coordination numbers of less than 4, represent a category of ubiquitous reactive intermediates in metal-catalyzed reactions that take place in solution, in metalloenzymes, on supported nanomaterials and single-atom catalysts, and so
on. While reactive intermediates are usually transient and hard to isolate, which makes detailed investigation challenging, molecular representatives of low-coordinate transition-metal intermediates can be synthesized by the judicious use of supporting ligands, allowing detailed study of their inherent chemical and physical properties. Aiming to enrich our knowledge on this category of transition-metal species, we have carried out a study of low-coordinate low-valent (-1; 0; +1) and high-valent (+4; +5) transition metal complexes, coordinatively unsaturated metal nitrogen complexes and low-coordinate metal-nitrogen multiple bond complexes. The successful isolation of these complexes would certainly push the boundary of low-coordinate transition-metal complexes chemistry.
Simple molecules O2, N2, CO2, and H2 are ubiquitous resources to human beings which can be used as energy reservoir to fuel the biological system or as chemical materials for the construction of complex molecules. The successful utilization of these small molecules relies on their effective activation since these molecules are quite thermodynamically stable. Despite the inertness of these molecules, many metallo-proteins and enzymes have been found efficient to mediate their activation, for example, [Fe,Fe]-hydrogenases can oxidize H2 to proton, Ni-dependent carbon monoxide dehydrogenases can promote the conversion of CO to CO2, and Fe, Mo-nitrogenases can reduce N2 to NH3, all under mild physiological conditions. Inspired by these enzymes, our solution to small molecules activation is to apply low-coordinate transition-metal complexes to coordinate with small molecules and subsequently facilitate their activation. Representative progresses include: 1) The synthesis and structural characterization of low-valent (-1; 0; +1) iron and cobalt dinitrogen complexes. 2) The establishment of the conversion of dinitrogen to 1,2-disilyldiazene compounds, which represents a new reaction mode of coordinated nitrogen.

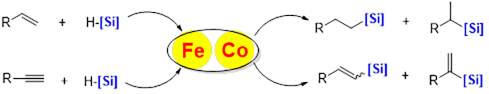
Organosilicon compounds are widely used in organic synthesis, polymer chemistry, and material science. Transition-metal-catalyzed hydrosilylation reaction has been regarded as one of the most straightforward and atom-economic approaches to obtain organosilicon compounds. Great achievements have been made by noble-metal-catalyst systems, such as Pt, Rh, Ru, Pd, and Ir in lab and industry. The non-precious, less toxic, environmentally more sustainable first-row transition metals, such as Fe and Co, have gain more attention. Research in our group also focuses on the development of structurally well-defined iron and cobalt complexes catalyzed hydrosilylation reaction of alkynes and alkenes with more efficient and active comparable to rare and precious transition metal catalytic system.